Is Used to Describe a Liquid That Evaporates Easily
As such it is one of the four fundamental states of matter the others being solid gas and plasma and is the only state with a definite volume but no fixed shapeA liquid is made up of tiny vibrating particles of. A volatile liquid one that easily bartleby.

Intermolecular Forces Demonstration Relative Evaporation Rates Of Volatile Liquids Chemdemos
A volatile liquid one that easily evaporates is put into a jar and the jar is then sealed.

. The liquid molecules escape into the gas phase becoming water vapor. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. Describe a liquid in terms of particle spacing.
Because the particles of a liquid are in constant motion they will collide with one. 39K views Quora User. A liquid with a high vapor pressure can evaporate easily and it is called volatile.
The tipical liquid treated is an aqueous waste with organic and inorganic pollutants having a concentration not greater then 100 gL. - Answers In chemistry thermodynamics and other areas we use the term volatility to speak to the characteric of a substance to easily vaporize. 1 Question 13 Which of these examples are liquids with high vapor pressure evaporates easily.
In evaporation a portion of the solvent is vaporized or boiled away leaving a thick liquid or solid precipitate as the final product. Evaporation is used to obtain a solid substance that has dissolved in water or any other liquid. The evaporation of a volatile liquid is an endothermic process that results in a temperature decrease.
In this equation h is the height of the liquid inside the capillary tube relative to the surface of the liquid outside the tube T is the surface tension of the liquid θ is the contact angle between the liquid and the tube r is the radius of the tube ρ is the density of the liquid and g is the acceleration due to gravity 98 ms 2When the tube is made of a material to which the liquid. View the full answer. Evaporation happens when a liquid substance becomes a gas.
When evaporating the rate of. When a solution containing dissolved solutes is heated the solvent will evaporate. A liquid with weak intermolecular forces evaporates more easily and has a high vapor pressure.
A liquid that evaporates easily has high vp and weak intermolecular forces. When water is heated it evaporates. The alcohol works because it evaporates quickly and lowers skin temperature.
It can be easily visualized when rain puddles disappear on a hot day or when wet clothes dry in the sun. Only the solute will remain in the evaporating dish. Evaporation is a change of phase from liquid to gas explained as follows.
In these examples the liquid water is not actually vanishingit is evaporating into a gas called water vapor. Evaporation may also be used as a method to produce a liquid or gaseous product obtained from the condensed vapor. A puddle of water left undisturbed eventually disappears.
Different types of evaporators can face different water treatment problem. Heat from the sun or solar energy powers the evaporation process. The vapor is condensed to recover the solvent or it can simply be discarded.
A liquid or hyper-granular is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. Water starts evaporating at 32 degrees F 0 degrees C but it. When you spray perfume on your body your body feels slightly cooler.
It soaks up moisture from. Evaporation of a substance is defined as the process that occurs when a liquid becomes a gas. Evaporation is the process when molecules from liquid pass to the atmosphere as gas without reaching the boiling point.
If the water is instead kept in a closed container the water vapor molecules do not have. This is an effect of evaporation or the change of matter from its liquid state to its vapour state. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.
The use of process of evaporation for separating a mixture is based on the fact that liquid vaporise easily whereas solids do not vaporise easily. A Motor oil maple syrup honey B Mercury water glycerin Alcohol vinegar acetone D Rubbing alcohol acetone Butane Propane Question 14 11 Which of these examples are liquids of low viscosity. Evaporation is a process during which water in a liquid state changes to gas or vapor.
When heated the particles of the liquid move faster allowing the liquid to flow more easily. Evaporation happens on a global scale. Water is a volatile substance meaning it evaporates easily.
What is a liquid that evaporates easily called. The dissolved substance is left as a solid residue when all the water has evaporated. Evaporation is a very important part of the water cycle.
The magnitude of temperature decrease is related to the strength of intermolecular forces of attraction. The rate of evaporation depends on the room temperature and a property of liquids called vapor pressure. Slow evaporation rate Has strong IM forces between particles so it requires MORE kinetic energy to become a.
Vocabulary Evaporation happens when a liquid turns into a gas. Eventually they have enough energy to escape the forces of. Vaporization is the process in which a liquid is converted to a gas.
Answered 5 years ago Author has 46K answers and 5M answer views All liquids evaporate except when in a closed container then no liquids evaporate. Furthermore temperature is defined to be a measure of the average kinetic energy of the particles. Many of the substances dissolves in water such as salts are not volatile and so get left behind when the water evaporates.
Evaporation can generally be defined as a process by which a liquid or solid is transformed into vapour. The vapor pressure of a substance at a given temperature is based on the strength of its intermolecular forces. The molecules of a substance must gain kinetic energy.
A Water alcohol gasoline B Rubbing. The same goes for acetone and water. A liquid with stronger intermolecular forces does not evaporate easily and thus has a lower vapor pressure.
If so you may have used rubbing alcohol to help cool your skin. Water and sugar 4 Evaporation to dryness - separate soluble solids from liquid When a solution is heated the solvent evaporates leaving behind the dissolved solids as residue. Describe the general shape of a liquid and use KMT to explain.
When a liquids vapor pressure equals atmospheric surrounding pressure At boiling where does the change from liquid to gas take place. Evaporation units or evaporators use the evaporation principle for the treatment of process water waste water and water based waste. Using KMT explain the evaporation rate of a volatile liquid.
When particles in the liquid phase are heated they gain kinetic energy and move faster and further apart. In the entire liquid not just the surface pockets of vapor appear as large bubbles What is a volatile liquid.
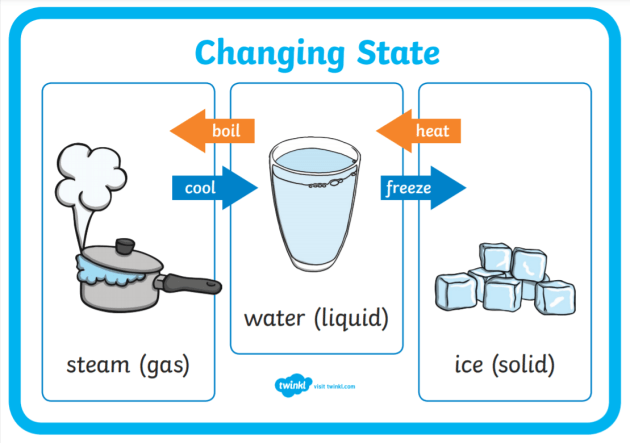
What Is Evaporation Definition Facts And Examples Twinkl
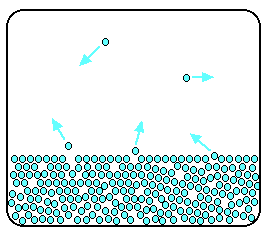
2 4 Vapor Pressure Chemistry Libretexts
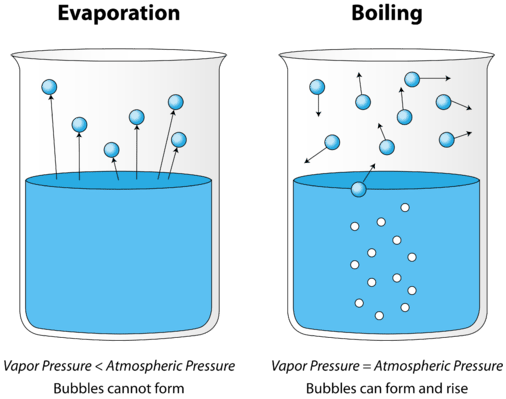
12 4 Evaporation And Condensation Chemistry Libretexts

Water Evaporation Experiment Youtube

Separation By Evaporation Geeksforgeeks

Intermolecular Forces Demonstration Relative Evaporation Rates Of Volatile Liquids Chemdemos
How Does Water Evaporate Below Its Boiling Point Quora
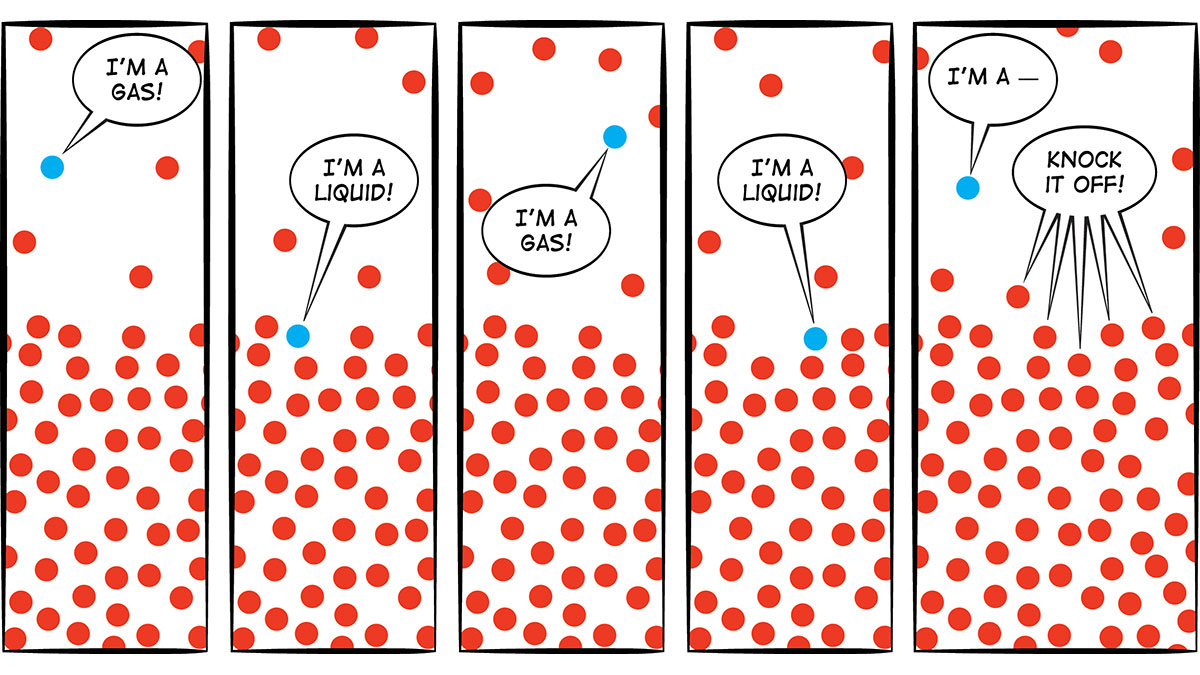
Q What S The Difference Between Evaporation And Boiling Nsta
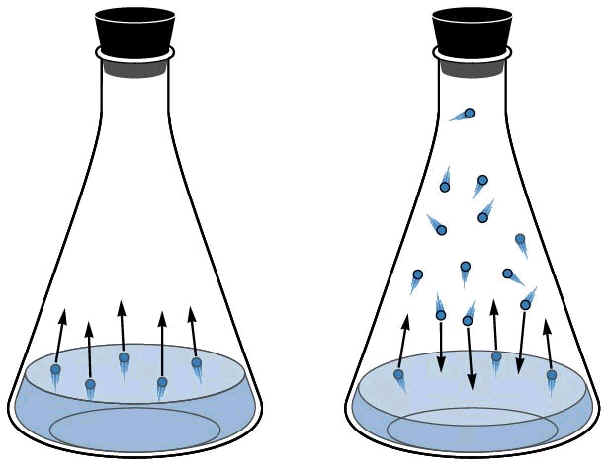
Everyday Chemistry What Happens To The Molecules Of A Liquid When It Evaporates Chemistry Stack Exchange
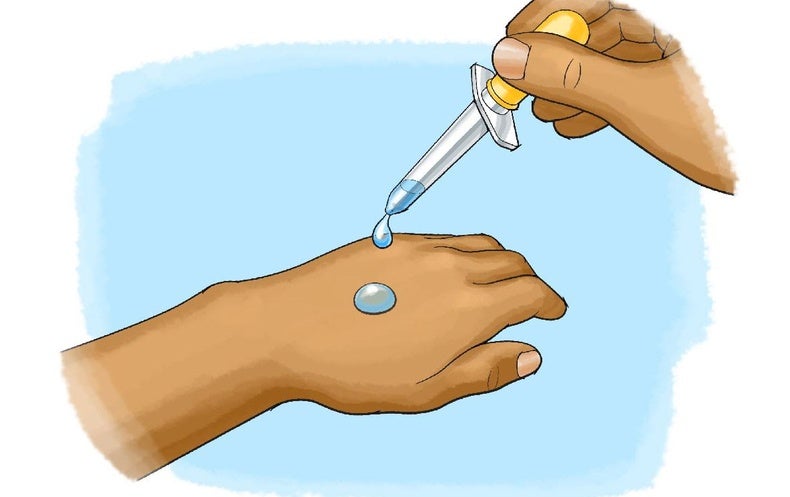
Chilling Science Evaporative Cooling With Liquids Scientific American
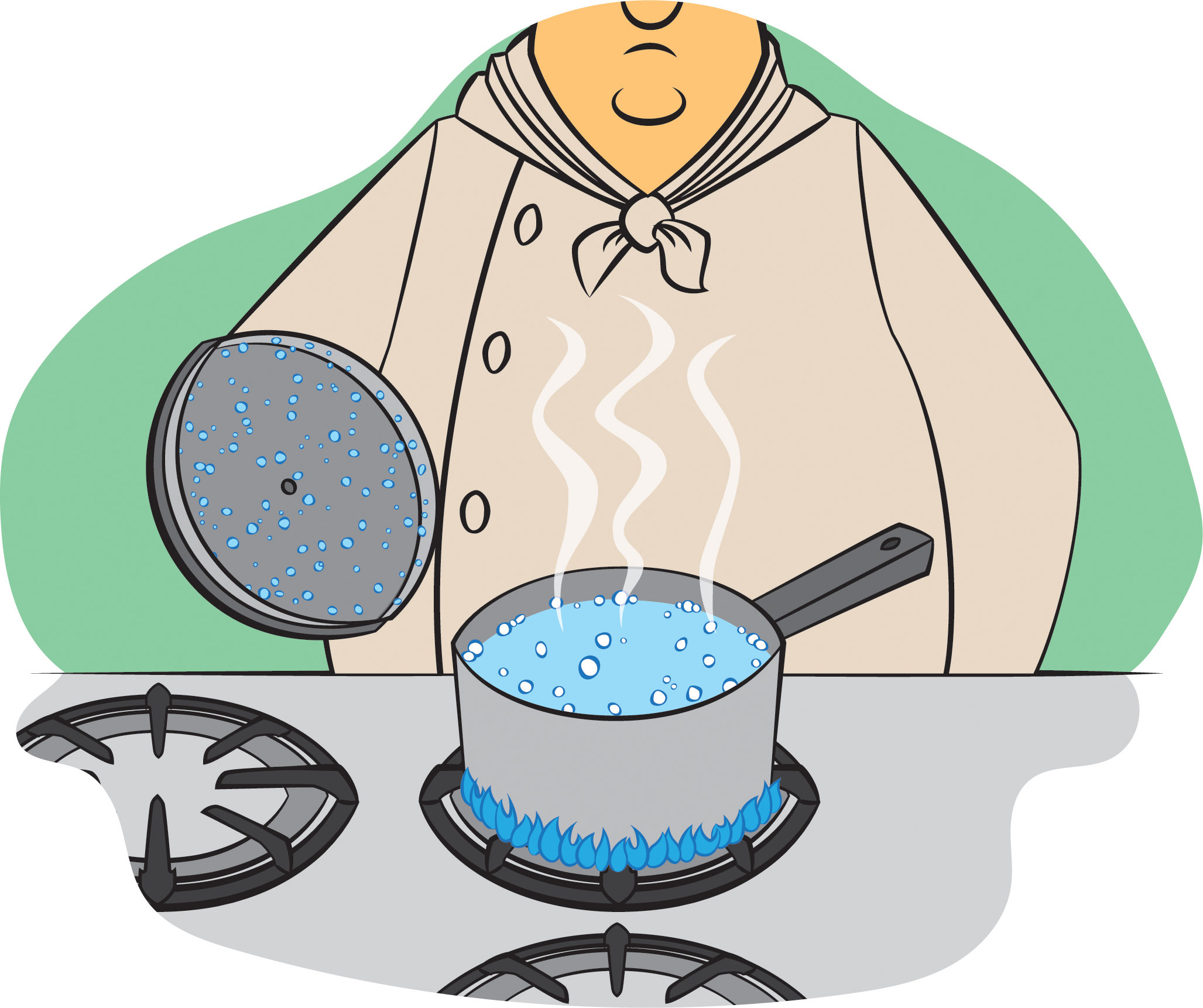
Q What S The Difference Between Evaporation And Boiling Nsta
Why Do Liquids Evaporate When They Aren T At Their Boiling Temperature Quora
Question Do All Liquids Evaporate At The Same Rate The Science Shop

Evaporation Chemistry For Non Majors
The Difference Between Evaporation And Distillation Difference Between

Evaporation Chemistry For Non Majors
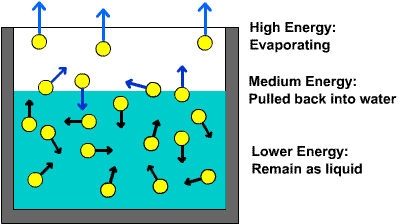
What Is Needed For Evaporation To Occur Explain In Terms Of Intermolecular Forces Socratic

Changing State Evaporation Chapter 2 States Of Matter Middle School Chemistry
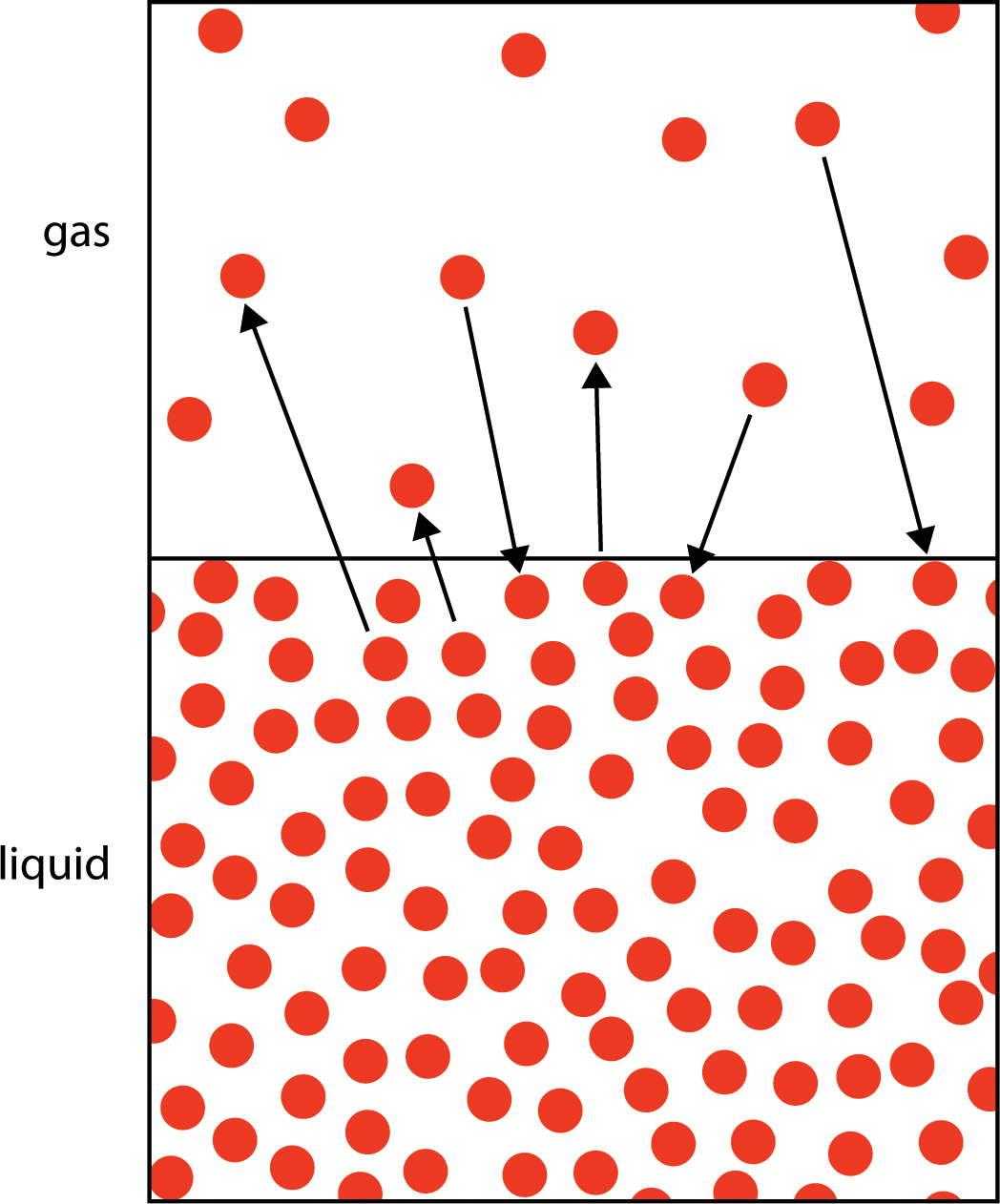
Q What S The Difference Between Evaporation And Boiling Nsta
Comments
Post a Comment